Citi Refresher Course
Citi Refresher Course - Peoria irb requires its irb chairs, members, alternates and researchers to complete the citi refresher courses every three years following the initial education program. How do i complete the refresher course? Human research curriculum (satisfies irb certification requirements, not rcr) refresher course (the basic course must have been completed previously). All uic investigators and key research personnel* are required to complete the initial investigator training requirements in human subject protections, a citi human subjects protections (hsp). After you log into the citi site, the. You are required to renew your citi training every three years by taking the relevant refresher course(s). The refresher courses are shorter than the original training course but will contain any new or revised information a researcher would need. Citi program provides training courses for colleges and universities, healthcare institutions, technology and research organizations, and governmental agencies. Listing of rcr refresher continuing education certified modules and requirements to apply for ce credits for this course Online rcr course through citi; Stanford provides access to required training through an interactive online tutorial, the citi. Then click the next button at the. After you have completed a basic or refresher course, citi will send the vanderbilt human research protection program (vhrpp) an email within. It covers historical and current information on regulatory. Required tutorial on human subject research protection and good clinical practice. Listing of rcr refresher continuing education certified modules and requirements to apply for ce credits for this course Gcp ich refresher offers retraining on gcp for clinical trials with investigational drugs and biologics (ich focus). All uic investigators and key research personnel* are required to complete the initial investigator training requirements in human subject protections, a citi human subjects protections (hsp). Human research curriculum (satisfies irb certification requirements, not rcr) refresher course (the basic course must have been completed previously). How do i complete the refresher course? After you have completed a basic or refresher course, citi will send the vanderbilt human research protection program (vhrpp) an email within. Required tutorial on human subject research protection and good clinical practice. If you remain engaged in human subject research, the citi training refresher course must be completed every three (3) years. How do i complete the refresher course?. Then click the next button at the. Citi program provides training courses for colleges and universities, healthcare institutions, technology and research organizations, and governmental agencies. If you remain engaged in human subject research, the citi training refresher course must be completed every three (3) years. It covers historical and current information on regulatory. The refresher courses are shorter than the. After you log into the citi site, the. After you have completed a basic or refresher course, citi will send the vanderbilt human research protection program (vhrpp) an email within. Learners will gain a solid understanding of how to design quantitative, qualitative, and mixed methods studies, collect and analyze data, and draw meaningful conclusions across various. Loyola university chicago uses. It covers historical and current information. Citi rcr refresher stage (complete once every 2 years) will i receive a reminder email to let me know my rcr training will soon expire? Required tutorial on human subject research protection and good clinical practice. All uic investigators and key research personnel* are required to complete the initial investigator training requirements in human. It covers historical and current information. How do i complete the refresher course? Stanford provides access to required training through an interactive online tutorial, the citi. All uic investigators and key research personnel* are required to complete the initial investigator training requirements in human subject protections, a citi human subjects protections (hsp). Citi rcr refresher stage (complete once every 2. After you have completed a basic or refresher course, citi will send the vanderbilt human research protection program (vhrpp) an email within. Required tutorial on human subject research protection and good clinical practice. Citi rcr refresher stage (complete once every 2 years) will i receive a reminder email to let me know my rcr training will soon expire? You are. Then click the next button at the. View details at citi program. After you have completed a basic or refresher course, citi will send the vanderbilt human research protection program (vhrpp) an email within. Gcp ich refresher offers retraining on gcp for clinical trials with investigational drugs and biologics (ich focus). All uic investigators and key research personnel* are required. If you remain engaged in human subject research, the citi training refresher course must be completed every three (3) years. You are required to renew your citi training every three years by taking the relevant refresher course(s). How do i complete the refresher course? It covers historical and current information. Learners will gain a solid understanding of how to design. Gcp ich refresher offers retraining on gcp for clinical trials with investigational drugs and biologics (ich focus). It covers historical and current information. Click on the link to complete the course. Learners will gain a solid understanding of how to design quantitative, qualitative, and mixed methods studies, collect and analyze data, and draw meaningful conclusions across various. Loyola university chicago. It covers historical and current information on regulatory. View details at citi program. Click on the link to complete the course. After you have completed a basic or refresher course, citi will send the vanderbilt human research protection program (vhrpp) an email within. Gcp ich refresher offers retraining on gcp for clinical trials with investigational drugs and biologics (ich focus). Stanford provides access to required training through an interactive online tutorial, the citi. Peoria irb requires its irb chairs, members, alternates and researchers to complete the citi refresher courses every three years following the initial education program. Citi program provides training courses for colleges and universities, healthcare institutions, technology and research organizations, and governmental agencies. It covers historical and current information. Loyola university chicago uses the online training course called the collaborative irb training initiative (citi) course to provide convenient web based training programs to the research. Gcp ich refresher offers retraining on gcp for clinical trials with investigational drugs and biologics (ich focus). Listing of rcr refresher continuing education certified modules and requirements to apply for ce credits for this course View details at citi program. After you have completed a basic or refresher course, citi will send the vanderbilt human research protection program (vhrpp) an email within. It covers historical and current information on regulatory. If you remain engaged in human subject research, the citi training refresher course must be completed every three (3) years. Required tutorial on human subject research protection and good clinical practice. Click on the link to complete the course. The refresher courses are shorter than the original training course but will contain any new or revised information a researcher would need. Human research curriculum (satisfies irb certification requirements, not rcr) refresher course (the basic course must have been completed previously). Citi rcr refresher stage (complete once every 2 years) will i receive a reminder email to let me know my rcr training will soon expire?Required Ethics Training Committee on the Use of Human Subjects
CITI Social Behavioral Science Basic/Refresher Training Access YouTube
Training & Certification Institutional Review Board Teachers
Reading and Understanding a CITI Program Completion Report and
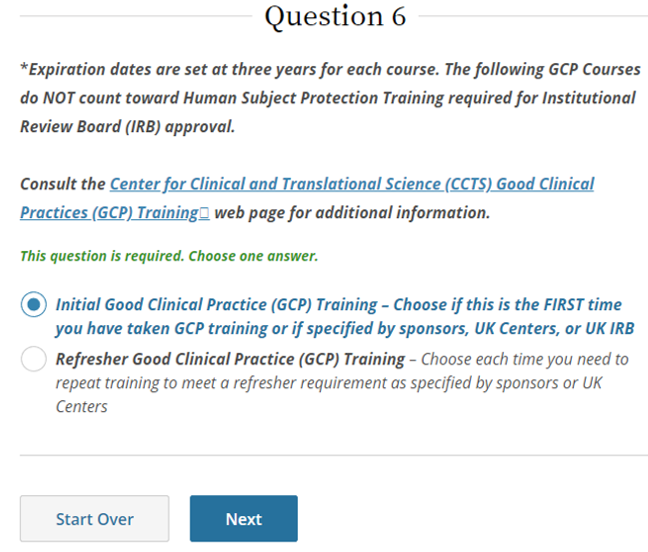
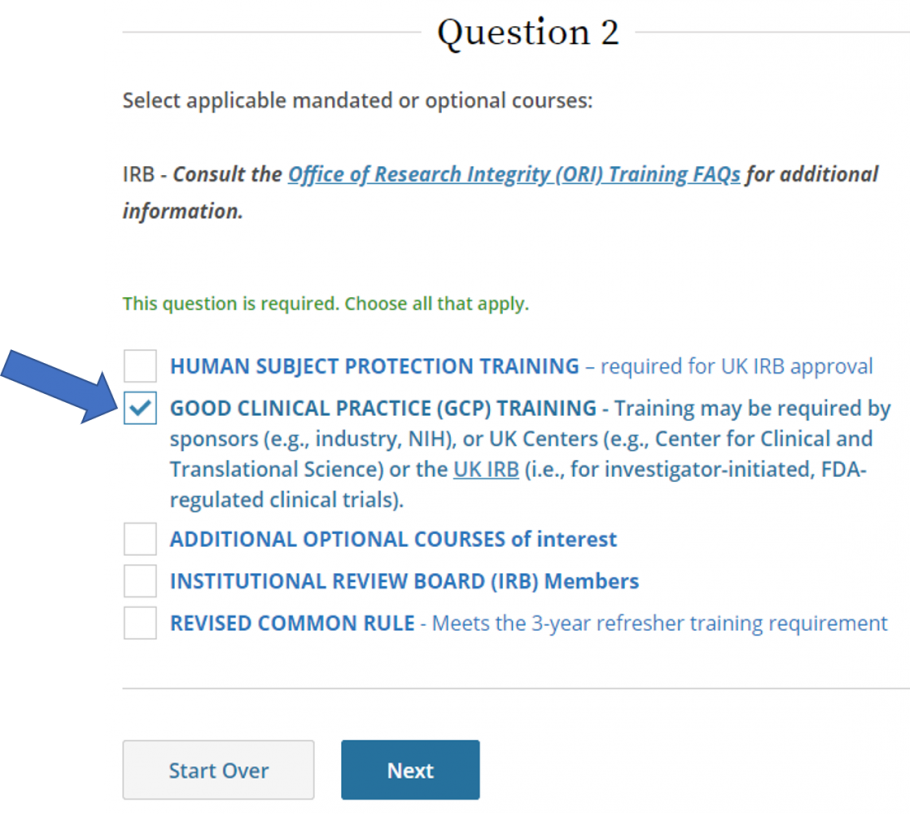
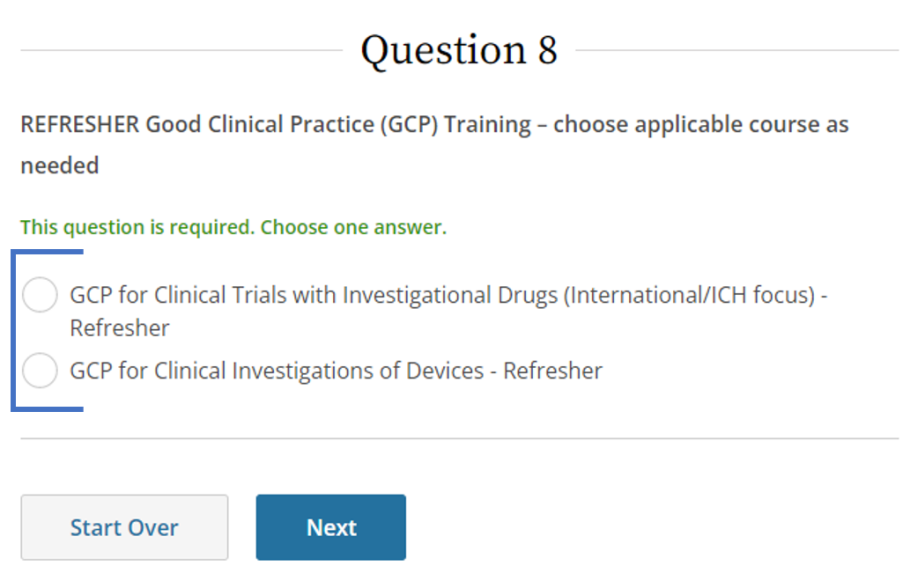
SponsorInvestigator (GCP) CITI Training FAQs University of Kentucky
SponsorInvestigator (GCP) CITI Training FAQs University of Kentucky
Citi Training GCP and Refresher Exam Questions with Correct Answers
Guide to GCP Mutual Recognition Completion Documentation
CITI Social and Behavioral Research Basic.Refresher Course PDF
SponsorInvestigator (GCP) CITI Training FAQs University of Kentucky
How Do I Complete The Refresher Course?
You Are Required To Renew Your Citi Training Every Three Years By Taking The Relevant Refresher Course(S).
Then Click The Next Button At The.
All Uic Investigators And Key Research Personnel* Are Required To Complete The Initial Investigator Training Requirements In Human Subject Protections, A Citi Human Subjects Protections (Hsp).
Related Post: