Medical Device Certification Courses
Medical Device Certification Courses - 8 june 2025 the world health organization (who) is seeking experts to serve as members of the strategic and technical advisory group on. Free online courses for state, local, and tribal regulatory. Prepmd students learn the clinical, technical, and business. Accurate classification of medical device risk levels is essential for regulatory oversight and clinical safety. Build a solid body of regulatory knowledge and advance your career with a raps online university certificate. Transfer friendlycourses designed for yourigorous curriculumlearn valuable skills This training will similarities and differences in. Medical sales college is a licensed private, postsecondary institution, training the next generation of medical sales reps. Learn about the medical device industry and regulations at your own. Online courses for industry on safety and effectiveness of medical devices and exposure to radiation from medical devices. Your company’s effectiveness depends on your employees’ skills and knowledge. Course tutors selected from the following: Document your dedication to medical devices by earning a professional certification from biopharma institute. Transfer friendlycourses designed for yourigorous curriculumlearn valuable skills Learn industry standards, requirements and best practices by our team of experts from the medical device industry. Medical device reprocessing acknowledged by ipac canada type: Medical sales college is a licensed private, postsecondary institution, training the next generation of medical sales reps. Meet the challenges of expanding regulations. How to register, etc., please contact. Transform you career with coursera's online medical device courses. Our medical device training and medical device quality assurance certification courses allow you to: Course tutors selected from the following: Learn fda cgmp regulations, quality system requirements, risk management, and compliance best. Accurate classification of medical device risk levels is essential for regulatory oversight and clinical safety. Earn a recognized certificate and cpd/ceu credits upon completion. Understand the fda classification system for medical devices. Analyze the regulatory requirements for class i, ii, and iii devices. Build a solid body of regulatory knowledge and advance your career with a raps online university certificate. Accurate classification of medical device risk levels is essential for regulatory oversight and clinical safety. The cbet certification validates expertise in testing, calibrating, and. Earn a recognized certificate and cpd/ceu credits upon completion. Transfer friendlycourses designed for yourigorous curriculumlearn valuable skills Accurate classification of medical device risk levels is essential for regulatory oversight and clinical safety. Online courses for industry on safety and effectiveness of medical devices and exposure to radiation from medical devices. The cbet certification validates expertise in testing, calibrating, and maintaining. Medical sales college is a licensed private, postsecondary institution, training the next generation of medical sales reps. Free online courses for state, local, and tribal regulatory. Meet the challenges of expanding regulations. Overview of our pharmaceutical, medical device, and biotechnology certificate programs categorized by industry. Understand the fda classification system for medical devices. Medical device reprocessing acknowledged by ipac canada type: Meet the challenges of expanding regulations. Accurate classification of medical device risk levels is essential for regulatory oversight and clinical safety. Learn about the medical device industry and regulations at your own. Analyze the regulatory requirements for class i, ii, and iii devices. Build a solid body of regulatory knowledge and advance your career with a raps online university certificate. Free online courses for state, local, and tribal regulatory. Online courses for industry on safety and effectiveness of medical devices and exposure to radiation from medical devices. Increase your understanding of specific topics shaping the medtech sector through our wide range of highly. Our medical device training and medical device quality assurance certification courses allow you to: We select talented and driven individuals for admissions into the prepmd cardiac medical device specialist training program. Your company’s effectiveness depends on your employees’ skills and knowledge. 8 june 2025 the world health organization (who) is seeking experts to serve as members of the strategic and. 8 june 2025 the world health organization (who) is seeking experts to serve as members of the strategic and technical advisory group on. This training will similarities and differences in. Transfer friendlycourses designed for yourigorous curriculumlearn valuable skills Prepmd students learn the clinical, technical, and business. Understand the fda classification system for medical devices. Welcome to the medical device single audit program (mdsap) compliance course. Increase your understanding of specific topics shaping the medtech sector through our wide range of highly specialized courses. Your company’s effectiveness depends on your employees’ skills and knowledge. Learn industry standards, requirements and best practices by our team of experts from the medical device industry. Prepmd students learn the. Welcome to the medical device single audit program (mdsap) compliance course. Medical sales college is a licensed private, postsecondary institution, training the next generation of medical sales reps. Meet the challenges of expanding regulations. Your company’s effectiveness depends on your employees’ skills and knowledge. Analyze the regulatory requirements for class i, ii, and iii devices. Accurate classification of medical device risk levels is essential for regulatory oversight and clinical safety. Transform you career with coursera's online medical device courses. 8 june 2025 the world health organization (who) is seeking experts to serve as members of the strategic and technical advisory group on. How to register, etc., please contact. Your company’s effectiveness depends on your employees’ skills and knowledge. This training will similarities and differences in. Our training covers risk management, pharmaceutical,. Learn about the medical device industry and regulations at your own. Build a solid body of regulatory knowledge and advance your career with a raps online university certificate. Free online courses for state, local, and tribal regulatory. Earn a recognized certificate and cpd/ceu credits upon completion. Learn industry standards, requirements and best practices by our team of experts from the medical device industry. Learn fda cgmp regulations, quality system requirements, risk management, and compliance best. The dual certificate program in medical devices and pharmaceuticals. We select talented and driven individuals for admissions into the prepmd cardiac medical device specialist training program. Increase your understanding of specific topics shaping the medtech sector through our wide range of highly specialized courses.Certification Of Medical Devices
Achieve More with Medical Device Training and Certifications Medical
Medical Arts Pharmacy Fayetteville Arkansas Medical Device Training
Medical Device Medical Representative Training Royed Training
Workforce Learning Solutions Hurix Digital
Medical Devices Compliance Advanced Training Artixio
Medical Device Training Courses Medical Device HQ
PPT ISO 13485 QMS Medical Device Certification PowerPoint
Medical Device Training Courses Medical Device HQ
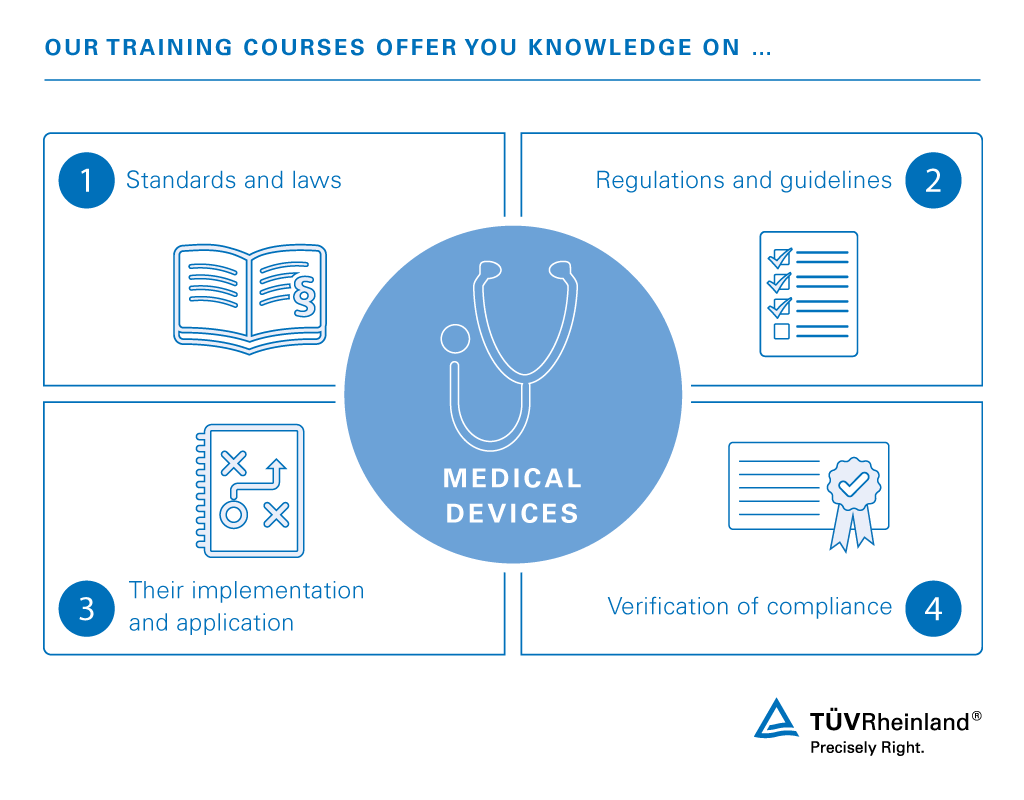
Medical Device Training TH TÜV Rheinland
The Cbet Certification Validates Expertise In Testing, Calibrating, And Maintaining Medical.
Medical Device Reprocessing Acknowledged By Ipac Canada Type:
Understand The Fda Classification System For Medical Devices.
Iso 14971 Risk Management Online Certified Course Designed For Medical Device Professionals.
Related Post: