Validation Courses
Validation Courses - Fda software validation guidelines training. Explore the vital role of validation, calibration, and qualification in pharmaceutical production and quality assurance. Elevate your engineering expertise with the “validation engineer mastery” training bundle. This elearning course aims to improve your knowledge and understanding of good manufacturing practice (gmp) requirements for validation life cycle, process validation,. Pharmaceutical & life science industry validation. This course is designed for product owners, product managers, business analysts and product teams to enable them to better incorporate discovery and validation into their product. Up to 10% cash back in this course, you will learn how to: Immediate access to training materials. Objectives include understanding validation principles, exploring calibration. For more information on these courses click here. Cyber threats evolve rapidly, and attackers don’t follow textbooks. Immediate access to training materials. Identify the purpose of data validation. For more information on these courses click here. This course is designed for product owners, product managers, business analysts and product teams to enable them to better incorporate discovery and validation into their product. Certificates validity can be checked online through our certificate online checker. This elearning course aims to improve your knowledge and understanding of good manufacturing practice (gmp) requirements for validation life cycle, process validation,. Elevate your engineering expertise with the “validation engineer mastery” training bundle. Gain insights into fda regulations with fda software validation guidelines. To learn data validation in excel, start by exploring its options under the data tab. The analytical method validation course is designed to provide a comprehensive understanding of method validation, regulatory requirements, and best practices in pharmaceutical analysis. Explore the vital role of validation, calibration, and qualification in pharmaceutical production and quality assurance. This elearning course aims to improve your knowledge and understanding of good manufacturing practice (gmp) requirements for validation life cycle, process validation,.. Cyber threats evolve rapidly, and attackers don’t follow textbooks. Objectives include understanding validation principles, exploring calibration. Explore the vital role of validation, calibration, and qualification in pharmaceutical production and quality assurance. This course is designed for product owners, product managers, business analysts and product teams to enable them to better incorporate discovery and validation into their product. To learn data. Cfpa offers numerous pharmaceutical validation courses: Certificates validity can be checked online through our certificate online checker. Troubleshoot common issues with data. Pharmaceutical & life science industry validation. Objectives include understanding validation principles, exploring calibration. Streamlined network access requests with the new account validation system (avs) starting april 25, 2025, the new account validation system (avs) will become the. Fda software validation guidelines training. Cfpa offers numerous pharmaceutical validation courses: Document your dedication to quality, compliance, safety, and job performance by earning a certification of training. Objectives include understanding validation principles, exploring calibration. Objectives include understanding validation principles, exploring calibration. For more information on these courses click here. Validation master planning, the validation of large capital expansion projects, technology transfers, the revision of systems in response to new regulations and. Up to 10% cash back in this course, you will learn how to: Document your dedication to quality, compliance, safety, and job performance. Troubleshoot common issues with data. To learn data validation in excel, start by exploring its options under the data tab. Document your dedication to quality, compliance, safety, and job performance by earning a certification of training. Streamlined network access requests with the new account validation system (avs) starting april 25, 2025, the new account validation system (avs) will become the.. Cyber threats evolve rapidly, and attackers don’t follow textbooks. To learn data validation in excel, start by exploring its options under the data tab. Elevate your engineering expertise with the “validation engineer mastery” training bundle. This elearning course aims to improve your knowledge and understanding of good manufacturing practice (gmp) requirements for validation life cycle, process validation,. Cfpa offers numerous. Elevate your engineering expertise with the “validation engineer mastery” training bundle. Cfpa offers numerous pharmaceutical validation courses: Explore the vital role of validation, calibration, and qualification in pharmaceutical production and quality assurance. Streamlined network access requests with the new account validation system (avs) starting april 25, 2025, the new account validation system (avs) will become the. Identify the purpose of. Cyber threats evolve rapidly, and attackers don’t follow textbooks. Streamlined network access requests with the new account validation system (avs) starting april 25, 2025, the new account validation system (avs) will become the. Identify the purpose of data validation. Validation training (online) to enhance regulatory compliance knowledge & qualification and validation skills. For more information on these courses click here. Immediate access to training materials. Objectives include understanding validation principles, exploring calibration. Troubleshoot common issues with data. Elevate your engineering expertise with the “validation engineer mastery” training bundle. This course is designed for product owners, product managers, business analysts and product teams to enable them to better incorporate discovery and validation into their product. Immediate access to training materials. Fda software validation guidelines training. This course is designed for product owners, product managers, business analysts and product teams to enable them to better incorporate discovery and validation into their product. Streamlined network access requests with the new account validation system (avs) starting april 25, 2025, the new account validation system (avs) will become the. Document your dedication to quality, compliance, safety, and job performance by earning a certification of training. The analytical method validation course is designed to provide a comprehensive understanding of method validation, regulatory requirements, and best practices in pharmaceutical analysis. Troubleshoot common issues with data. Validation training (online) to enhance regulatory compliance knowledge & qualification and validation skills. Gain insights into fda regulations with fda software validation guidelines. Elevate your engineering expertise with the “validation engineer mastery” training bundle. Explore the vital role of validation, calibration, and qualification in pharmaceutical production and quality assurance. Up to 10% cash back in this course, you will learn how to: Identify the purpose of data validation. This elearning course aims to improve your knowledge and understanding of good manufacturing practice (gmp) requirements for validation life cycle, process validation,. Objectives include understanding validation principles, exploring calibration. Validation master planning, the validation of large capital expansion projects, technology transfers, the revision of systems in response to new regulations and.Train Test Validation Split How To & Best Practices [2023]
Training on Model Validation
Concept Summary Model Validation — Dataiku Knowledge Base
Machine Learning Training, Validation & Test Data Set Analytics Yogi
Train Test Validation Split How To & Best Practices [2023]
A Brief Idea on Online Computer System Validation Training Courses by
What is Assessment Validation? How to Validate Assessment Tools 360RTO
Process Validation Requirements and Industry Practices AAMI
Validation ISPE International Society for Pharmaceutical Engineering
Validation Technical Training Video AD YouTube
Cfpa Offers Numerous Pharmaceutical Validation Courses:
Cyber Threats Evolve Rapidly, And Attackers Don’t Follow Textbooks.
Pharmaceutical & Life Science Industry Validation.
For More Information On These Courses Click Here.
Related Post:
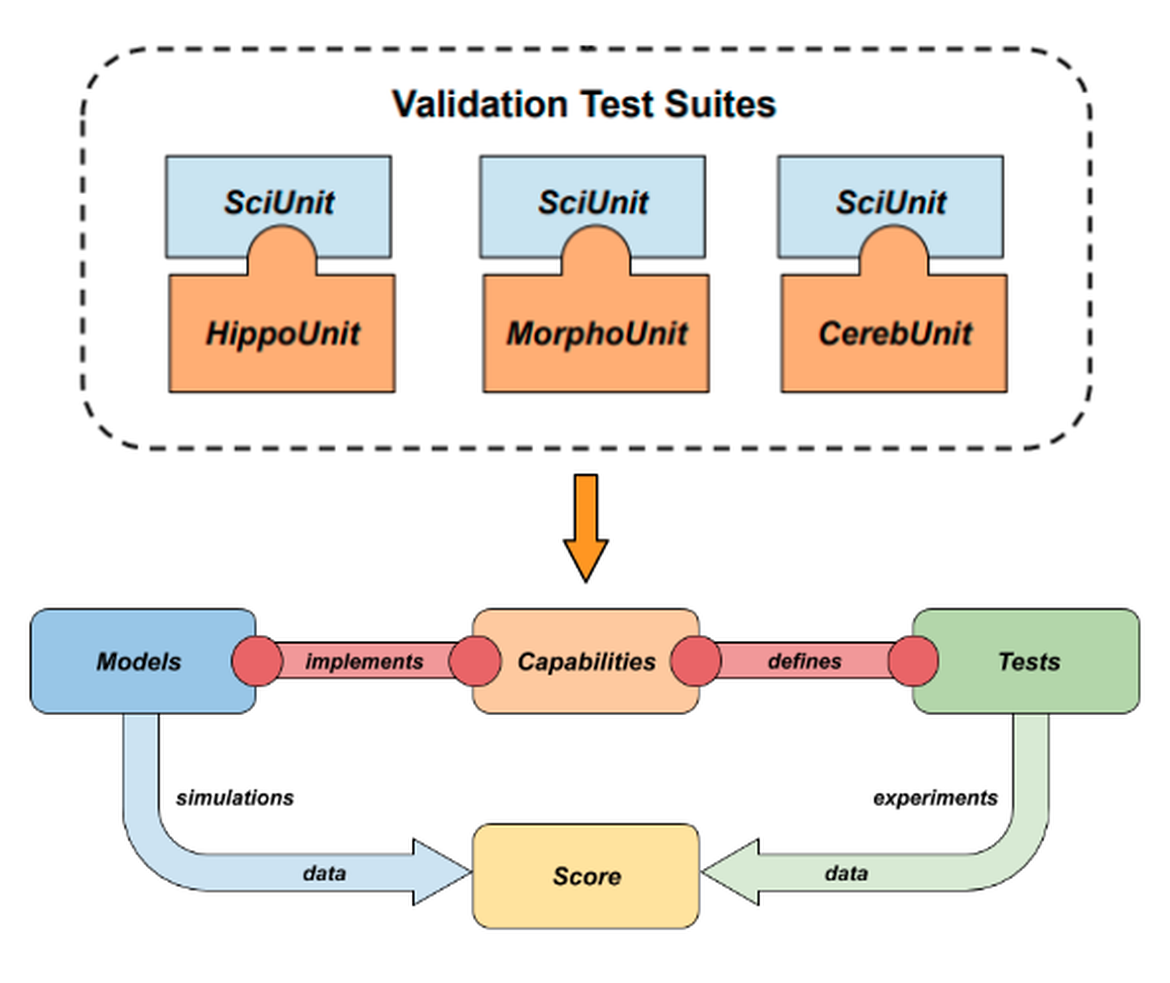
![Train Test Validation Split How To & Best Practices [2023]](https://assets-global.website-files.com/5d7b77b063a9066d83e1209c/613ec5b6c3da5313e1abcc47_UeKfm9v6E9QobwFfG3ud_20Q82QoqI8W6kXQnDm_QBnOVyQXCNmwjWtMI5vD9du4cjovnpzSYBbIDHdSU-57H1Bb4DfuUCaSjZjozKIwD0IQsH7FyMuFTW7aYVW-zelk2RNMAez3%3Ds0.png)
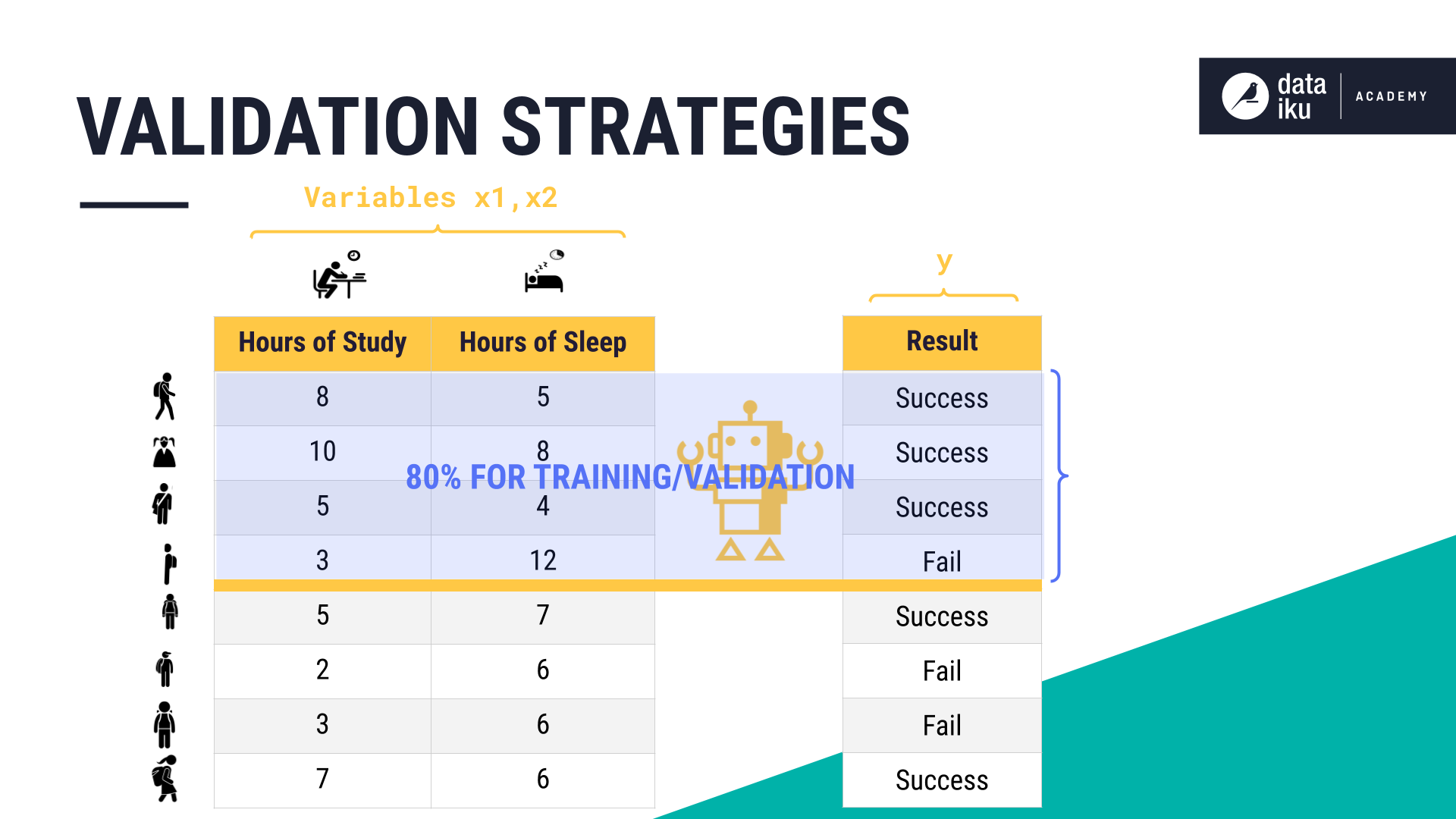
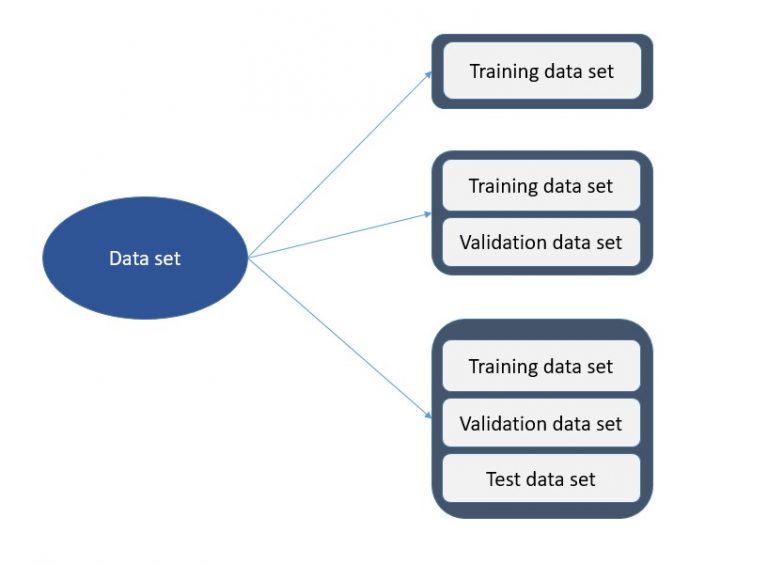
![Train Test Validation Split How To & Best Practices [2023]](https://assets-global.website-files.com/5d7b77b063a9066d83e1209c/61568656a13218cdde7f6166_training-data-validation-test.png)




